NH3 Intermolecular Forces
Hydrogen bonds, dipole-dipole interaction, and London dispersion forces are all parts of the nh3 intermolecular forces. These forces are present between nh3 molecules. Intermolecular interactions produced by nh3 molecules include hydrogen bonds, dipole dipole interaction, and London dispersion forces.
NH3 molecules generate intermolecular hydrogen bonding, dipole-dipole interaction, and London dispersion forces. Each force affects the molecule in different ways. We will discuss each of these forces in detail in this article. For more information, see the links below. This article will help you to understand the forces that NH3 molecules generate. So the next time you think of solving a chemical problem, think about how these forces work.
NH3 forms hydrogen bonds
The strongest of the intermolecular forces that bind molecules together is hydrogen bonding. In addition to hydrogen bonding, nh3 molecules exhibit dipole-dipole interaction and London dispersion forces. These forces, primarily responsible for NH3’s solid or liquid state, can also be described as the same kind of attraction. In the case of NH3, these forces are responsible for hydrogen bonding because of the electronegativity difference between the two atoms.
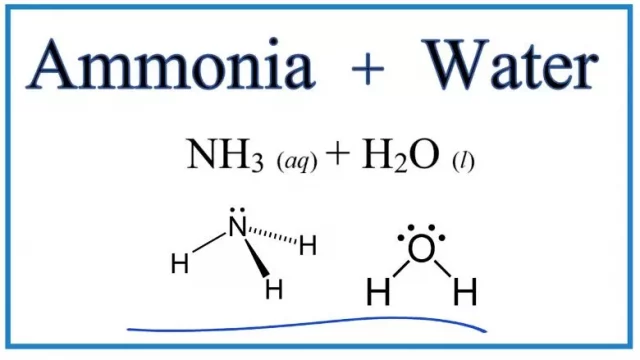
To establish a hydrogen bond, an nh3 molecule has a significant electronegativity difference compared to the hydrogen atom. The difference in electronegativity between the two elements is 0.8. A hydrogen atom has a partial positive charge that cannot be satisfied by its lone electron. Therefore, it forms a hydrogen bond with the water molecules. Thus, ammonia is easily soluble in water.

Ammonia’s hydrogen bonding ability is attributed to the weak intermolecular forces that it generates between its molecules. Hydrogen bonds are stronger between molecules with hydrogen atoms that are covalently bonded to each other. Hydrogen bonds are a more potent force among chemicals with high boiling points. So, read on if you want to know how hydrogen bonds are formed!
The intermolecular force between two dipoles or ions directly results from the attraction between the two atoms. This attractive energy is proportional to their molar masses. Therefore, higher polarities and molar masses are associated with higher boiling points. In the case of NH3, the differences in polarity will help you determine how strong hydrogen bonds are among the three NH3 compounds.
In liquid water, the two molecules can form hydrogen bonds because each hydrogen atom has a higher electronegative charge than the other. This is why NH3 forms two hydrogen bonds with water at a time. The two molecules are similar in molecular mass, but their boiling points are much lower than those of water. This makes them attractive to each other. This attraction is one of the primary reasons why these compounds are so important in biological systems.
NH3 has dipole-dipole force
Ammonia molecules have intermolecular forces: hydrogen bonding, dipole-dipole interaction, and London dispersion. Hydrogen and nitrogen have highly electronegative values, which is why they form a hydrogen bond. In addition, NH3 molecules have two kinds of hydrogen bonds: covalent and ionic. The London dispersion force acts on atoms in opposite directions, while the dipole-dipole attraction forces act only within one molecule.

Hydrogen bonding is more vital than hydrogen bonding between ammonia and water. Because oxygen is more electronegative than nitrogen, it has two lone electron pairs. These lone pairs cannot satisfy the partial positive charge on hydrogen atoms. Thus, NH3 forms hydrogen bonds with water molecules. This is the reason why ammonia dissolves in water so quickly. But how does the dipole-dipole force work?
Dipole-dipole forces are generated when a molecule has two poles, one partial positive and one negative. These polar molecules attract each other through dipole-dipole interaction. On the other hand, hydrogen bonds are formed between polar molecules. In water, the positive pole of one molecule attracts the negative pole of the other molecule. The opposite is true with ONF.
NH3 exhibits dipole-dipole force. The three hydrogen atoms are highly electronegative, and the middle atom is positively charged. This difference in electronegativity causes the NH3 molecule to exhibit polarity. Hence, the NH3 molecule is polar. This property results from the unequal sharing of electrons among the two atoms.
Dipole-dipole forces are the most potent intermolecular attraction between NH3 and H2O. However, dipole-dipole forces are only a part of the equation for ammonia. The hydrogen bonding between the NH3 and H2O molecules is much stronger than dipole-dipole forces. A stronger hydrogen bond between these two molecules can cause the ammonia to boil more quickly.
The NH3 molecule contains three covalent bonds, one with nitrogen and two with hydrogen. The molecule also has one unpaired electron, making the NH3 molecule more electronegative than H2 or H2S. Because the two compounds are electronegative, the boiling point of the two molecules is higher. In addition, a strong hydrogen bond between two atoms can also cause a high boiling point.
NH3 has London dispersion forces
In addition to hydrogen bonding, NH3 exhibits intermolecular forces, known as dipole-dipole interaction and London dispersion forces. The hydrogen-nitrate molecule is directly connected to nitrogen by hydrogen bonding, which is generated when one atom’s partial positive charge attracts another’s negative pole. The intermolecular forces between NH3 molecules are weak and governed by the covalent, ionic, and coordination bonds.
These forces act on every molecule, regardless of its type. The strength of London dispersion forces increases with the number of electrons in the molecule. This explains why bromine has a higher boiling point than chlorine, while chlorine’s is much lower. The London dispersion forces of the ammonia molecule are the most significant in chlorine, whereas they are relatively weak in NH3.
Another difference between ammonia and phosphine is the strength of their hydrogen bonds. Ammonia, on the other hand, has a higher boiling point than phosphine. Therefore, it is easier to calculate its boiling point. Hydrogen bonds are also more substantial than dipole-dipole forces, meaning the chemical will have a higher boiling point. The chemical properties of NH3 are the strongest of all the three types of ammonia, but this doesn’t explain why NH3 is more stable than phosphine.
Although London dispersion forces are weak in ammonia, the intermolecular force between ammonia and nitrate is much more vital. Thus, the boiling point of ammonia is higher than that of nitrates. In addition, the higher the intermolecular force, the higher the boiling point. But, the higher the boiling point, the higher the molecular energy needed to separate the two molecules.
Despite its weaker London dispersion forces, ammonia is still more electronegative than iodine. Although ammonia is a gas at room temperature, iodine is solid at the same temperature—moreover, ammonia exhibits all three vans der Waals forces. Learn more about liquids and gases and their properties. You’ll be glad you did.
NH3 has ion force
Hydrogen atoms directly bond to molecules with high electronegative values, such as nitrogen. These molecules then generate hydrogen bonds between themselves. The attraction between hydrogen and nitrogen is due to the significant difference in electronegativity between the two elements. This attraction is responsible for the formation of hydrogen bonds. This article explains how these bonds are formed and how this chemical bonding process affects the properties of NH3 molecules.
Hydrogen bonding occurs between hydrogen atoms and highly electronegative molecules like oxygen and fluorine. The strength of hydrogen bonding varies depending on the molecules’ electronegativity and size. For example, water has four hydrogen bonds while NH3 forms only one. These forces are the most critical aspects of hydrogen bonding. Hydrogen bonds are essential for proteins, nucleic acids, and many other biological molecules.
Another mechanism that can cause interactions between nonpolar molecules is dispersion. Electrostatic attraction occurs when electrons move in an opposite direction. Electrostatic attraction is minor and transient and depends on the number of electrons surrounding the nucleus. The stronger the attraction, the larger the molecule is, and the fewer the number of electrons. On the other hand, Helium has only two electrons and can only liquefy at 4.2 K.
NH3 Intermolecular Forces
Hydrogen bonds, dipole-dipole interaction, and London dispersion forces are all parts of the nh3 intermolecular forces. These forces are present between nh3 molecules. Intermolecular interactions produced by nh3 molecules include hydrogen bonds, dipole dipole interaction, and London dispersion forces.
NH3 molecules generate intermolecular hydrogen bonding, dipole-dipole interaction, and London dispersion forces. Each force affects the molecule in different ways. We will discuss each of these forces in detail in this article. For more information, see the links below. This article will help you to understand the forces that NH3 molecules generate. So the next time you think of solving a chemical problem, think about how these forces work.
NH3 forms hydrogen bonds
The strongest of the intermolecular forces that bind molecules together is hydrogen bonding. In addition to hydrogen bonding, nh3 molecules exhibit dipole-dipole interaction and London dispersion forces. These forces, primarily responsible for NH3’s solid or liquid state, can also be described as the same kind of attraction. In the case of NH3, these forces are responsible for hydrogen bonding because of the electronegativity difference between the two atoms.
To establish a hydrogen bond, an nh3 molecule has a significant electronegativity difference compared to the hydrogen atom. The difference in electronegativity between the two elements is 0.8. A hydrogen atom has a partial positive charge that cannot be satisfied by its lone electron. Therefore, it forms a hydrogen bond with the water molecules. Thus, ammonia is easily soluble in water.

Ammonia’s hydrogen bonding ability is attributed to the weak intermolecular forces that it generates between its molecules. Hydrogen bonds are stronger between molecules with hydrogen atoms that are covalently bonded to each other. Hydrogen bonds are a more potent force among chemicals with high boiling points. So, read on if you want to know how hydrogen bonds are formed!
The intermolecular force between two dipoles or ions directly results from the attraction between the two atoms. This attractive energy is proportional to their molar masses. Therefore, higher polarities and molar masses are associated with higher boiling points. In the case of NH3, the differences in polarity will help you determine how strong hydrogen bonds are among the three NH3 compounds.
In liquid water, the two molecules can form hydrogen bonds because each hydrogen atom has a higher electronegative charge than the other. This is why NH3 forms two hydrogen bonds with water at a time. The two molecules are similar in molecular mass, but their boiling points are much lower than those of water. This makes them attractive to each other. This attraction is one of the primary reasons why these compounds are so important in biological systems.
NH3 has dipole-dipole force
Ammonia molecules have intermolecular forces: hydrogen bonding, dipole-dipole interaction, and London dispersion. Hydrogen and nitrogen have highly electronegative values, which is why they form a hydrogen bond. In addition, NH3 molecules have two kinds of hydrogen bonds: covalent and ionic. The London dispersion force acts on atoms in opposite directions, while the dipole-dipole attraction forces act only within one molecule.

Hydrogen bonding is more vital than hydrogen bonding between ammonia and water. Because oxygen is more electronegative than nitrogen, it has two lone electron pairs. These lone pairs cannot satisfy the partial positive charge on hydrogen atoms. Thus, NH3 forms hydrogen bonds with water molecules. This is the reason why ammonia dissolves in water so quickly. But how does the dipole-dipole force work?
Dipole-dipole forces are generated when a molecule has two poles, one partial positive and one negative. These polar molecules attract each other through dipole-dipole interaction. On the other hand, hydrogen bonds are formed between polar molecules. In water, the positive pole of one molecule attracts the negative pole of the other molecule. The opposite is true with ONF.
NH3 exhibits dipole-dipole force. The three hydrogen atoms are highly electronegative, and the middle atom is positively charged. This difference in electronegativity causes the NH3 molecule to exhibit polarity. Hence, the NH3 molecule is polar. This property results from the unequal sharing of electrons among the two atoms.
Dipole-dipole forces are the most potent intermolecular attraction between NH3 and H2O. However, dipole-dipole forces are only a part of the equation for ammonia. The hydrogen bonding between the NH3 and H2O molecules is much stronger than dipole-dipole forces. A stronger hydrogen bond between these two molecules can cause the ammonia to boil more quickly.
The NH3 molecule contains three covalent bonds, one with nitrogen and two with hydrogen. The molecule also has one unpaired electron, making the NH3 molecule more electronegative than H2 or H2S. Because the two compounds are electronegative, the boiling point of the two molecules is higher. In addition, a strong hydrogen bond between two atoms can also cause a high boiling point.
NH3 has London dispersion forces
In addition to hydrogen bonding, NH3 exhibits intermolecular forces, known as dipole-dipole interaction and London dispersion forces. The hydrogen-nitrate molecule is directly connected to nitrogen by hydrogen bonding, which is generated when one atom’s partial positive charge attracts another’s negative pole. The intermolecular forces between NH3 molecules are weak and governed by the covalent, ionic, and coordination bonds.
These forces act on every molecule, regardless of its type. The strength of London dispersion forces increases with the number of electrons in the molecule. This explains why bromine has a higher boiling point than chlorine, while chlorine’s is much lower. The London dispersion forces of the ammonia molecule are the most significant in chlorine, whereas they are relatively weak in NH3.
Another difference between ammonia and phosphine is the strength of their hydrogen bonds. Ammonia, on the other hand, has a higher boiling point than phosphine. Therefore, it is easier to calculate its boiling point. Hydrogen bonds are also more substantial than dipole-dipole forces, meaning the chemical will have a higher boiling point. The chemical properties of NH3 are the strongest of all the three types of ammonia, but this doesn’t explain why NH3 is more stable than phosphine.
Although London dispersion forces are weak in ammonia, the intermolecular force between ammonia and nitrate is much more vital. Thus, the boiling point of ammonia is higher than that of nitrates. In addition, the higher the intermolecular force, the higher the boiling point. But, the higher the boiling point, the higher the molecular energy needed to separate the two molecules.
Despite its weaker London dispersion forces, ammonia is still more electronegative than iodine. Although ammonia is a gas at room temperature, iodine is solid at the same temperature—moreover, ammonia exhibits all three vans der Waals forces. Learn more about liquids and gases and their properties. You’ll be glad you did.
NH3 has ion force
Hydrogen atoms directly bond to molecules with high electronegative values, such as nitrogen. These molecules then generate hydrogen bonds between themselves. The attraction between hydrogen and nitrogen is due to the significant difference in electronegativity between the two elements. This attraction is responsible for the formation of hydrogen bonds. This article explains how these bonds are formed and how this chemical bonding process affects the properties of NH3 molecules.
Hydrogen bonding occurs between hydrogen atoms and highly electronegative molecules like oxygen and fluorine. The strength of hydrogen bonding varies depending on the molecules’ electronegativity and size. For example, water has four hydrogen bonds while NH3 forms only one. These forces are the most critical aspects of hydrogen bonding. Hydrogen bonds are essential for proteins, nucleic acids, and many other biological molecules.
Another mechanism that can cause interactions between nonpolar molecules is dispersion. Electrostatic attraction occurs when electrons move in an opposite direction. Electrostatic attraction is minor and transient and depends on the number of electrons surrounding the nucleus. The stronger the attraction, the larger the molecule is, and the fewer the number of electrons. On the other hand, Helium has only two electrons and can only liquefy at 4.2 K.