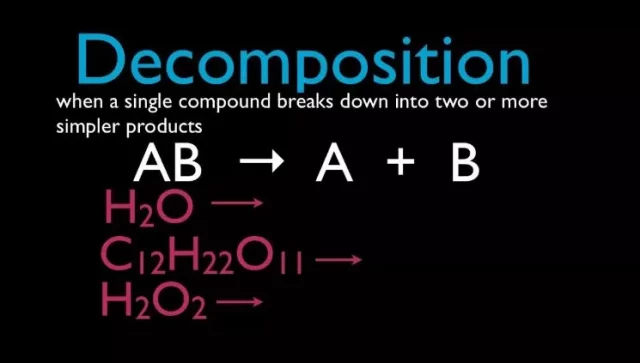The Balanced Equation For Decomposition of Hydrogen Peroxide
When one reactant decomposes into two or more products, the reaction is called a decomposition reaction. This can be modelled by the all-inclusive formula AB! A + B. The conversion of water to hydrogen and oxygen and the breakdown of hydrogen peroxide are two examples of decomposition reactions.
2 H2O2 → 2 H2O + O.
The balanced equation for the decomposition of hydrogen peroxide includes the name of the reactant, heat, and the catalyst used in the demonstration. The heat should indicate whether the reaction is endothermic or exothermic. The catalyst is also called a hydrogen peroxide catalyst in the demonstration. Therefore, it should also be included in the equation. The reaction can take place in a closed or open system.
Disproportionation reaction
Hydrogen peroxide undergoes a disproportionation reaction. In this reaction, oxygen is oxidized to a negative state while the other components are reduced to an intermediate oxidation state. In the same reaction, an element can become oxidized and reduced simultaneously. Disproportionation also occurs between a substance with an oxidation state of one and an inorganic substance with a lower oxidation state.
Hydrogen peroxide can be decomposed into mercuric chloride and sodium chloride by reacting with an alkaline solution. This reaction creates sodium chlorite and chloride, two elements with oxidation states of 0 and +5. The hydrogen peroxide gas reacts with the concentrated alkali to form chlorine halide and sodium chlorite. Mercury (Hg) is formed from mercuric chloride when the reaction is exposed to ultraviolet light.
The disproportionation reaction of hydrogen peroxide occurs when hydrogen is added to a solution of the substance. The addition of hydrogen decreases the high dose limiting concentration of hydrogen peroxide. The amount of hydrogen added decreases linearly with the concentration of hydrogen. However, when hydrogen is added to a solution of hydrogen peroxide, its effect on the decomposition rate is less clear.

Another way of determining hydrogen peroxide concentrations is by performing a putative disproportionation reaction. This method uses a sulfite-reducing bacteria called ATP sulfurylase. This method of hydrogen peroxide decomposition is much more effective in determining molecular weight and structure. In this way, the reaction can be monitored continuously, allowing the researcher to compare the hydrogen peroxide rate with ammonia.
Disproportionation reaction in hydrogen peroxide
The disproportionation reaction in hydrogen peroxide is the process by which H2O2 is reacted with univalent oxygen to form water and oxygen. The oxygen atoms in hydrogen peroxide are in an oxidation state of -1, so they can either be oxidized to form O2 or reduced to water. The overall cell potential for the disproportionation reaction is positive, so this is the appropriate mechanism for the decomposition of H2O2.
The disproportionation reaction can be further broken down into the oxidation and reduction half-reactions. This is done to determine how many electrons are involved in each half-reaction. The oxidation half must be multiplied by 2 to achieve balance. The reduction half will involve 3 molecules and four electrons. Once you know the number of electrons involved in each half-reaction, you can calculate the balance of oxidation and reduction.
Effects of catalyst on the decomposition of hydrogen peroxide
The redox properties of hydrogen peroxide are well-known, as they can remove electrons from other substances and reduce inorganic ions. In its natural form, hydrogen peroxide decomposes into water and oxygen, releasing free radicals. The decomposition reaction occurs in the presence of a catalyst, a transition metal. Transition metals such as iron and manganese are found in nearly every living creature.
The mineralogical composition of the terrigenous rock plays a crucial role in decomposing hydrogen peroxide. Catalytic features of metal surfaces also play an essential role. Lastly, the salinity of the water plays a vital role in the process, as it enhances the pressure of the hydrogen peroxide. Hydrogen peroxide decomposition results in a significant increase in pressure within a reservoir.
A catalyst is needed to increase the rate of hydrogen peroxide decomposition. It is not part of the products of the chemical reaction but instead helps it happen faster. The catalyst does not change during the reaction, but it speeds it up by increasing the reaction rate. As a result, oxygen gas is released from hydrogen peroxide, and the catalyst helps the reaction take place faster.
The effects of catalysts on hydrogen peroxide degradation depend highly on the type of substrate used and the reaction conditions. Aluminum has a high starting activity, while tin decreases inhibition. The concentration of these metals significantly affects the rate of decomposition of hydrogen peroxide. The concentration of the catalyst determines the degree of inhibition and the start of the activity.
While using an elephant toothpaste demonstration, the catalyst helps to speed up the chemical reaction. The elephant toothpaste, for example, produces water vapor. The decomposition process is slow, but the presence of a catalyst speeds it up significantly. Likewise, adding a small amount of soap to the mixture speeds up the process, producing a large amount of foam. However, it is essential to include yeast in the product side of the chemical equation.
Several different types of catalysts have been identified as potential candidates for hydrogen peroxide decomposition. Potassium iodide is an example of a material that is a suitable catalyst. Potassium iodide is a catalyst because it produces the hypoiodite ion when it reacts with hydrogen peroxide and creates water.
The decomposition of H2O2 can be controlled by adjusting several variables. Temperature, concentration, pH, and the type of catalyst all significantly impact the final product. These variables are essential when considering the decomposition of H2O2 in the oil phase. However, the rate at which hydrogen peroxide enters the oil phase is limited. The higher the concentration of H2O2, the slower the decomposition process takes place.
The Balanced Equation For Decomposition of Hydrogen Peroxide
When one reactant decomposes into two or more products, the reaction is called a decomposition reaction. This can be modelled by the all-inclusive formula AB! A + B. The conversion of water to hydrogen and oxygen and the breakdown of hydrogen peroxide are two examples of decomposition reactions.
2 H2O2 → 2 H2O + O.
The balanced equation for the decomposition of hydrogen peroxide includes the name of the reactant, heat, and the catalyst used in the demonstration. The heat should indicate whether the reaction is endothermic or exothermic. The catalyst is also called a hydrogen peroxide catalyst in the demonstration. Therefore, it should also be included in the equation. The reaction can take place in a closed or open system.
Disproportionation reaction
Hydrogen peroxide undergoes a disproportionation reaction. In this reaction, oxygen is oxidized to a negative state while the other components are reduced to an intermediate oxidation state. In the same reaction, an element can become oxidized and reduced simultaneously. Disproportionation also occurs between a substance with an oxidation state of one and an inorganic substance with a lower oxidation state.
Hydrogen peroxide can be decomposed into mercuric chloride and sodium chloride by reacting with an alkaline solution. This reaction creates sodium chlorite and chloride, two elements with oxidation states of 0 and +5. The hydrogen peroxide gas reacts with the concentrated alkali to form chlorine halide and sodium chlorite. Mercury (Hg) is formed from mercuric chloride when the reaction is exposed to ultraviolet light.
The disproportionation reaction of hydrogen peroxide occurs when hydrogen is added to a solution of the substance. The addition of hydrogen decreases the high dose limiting concentration of hydrogen peroxide. The amount of hydrogen added decreases linearly with the concentration of hydrogen. However, when hydrogen is added to a solution of hydrogen peroxide, its effect on the decomposition rate is less clear.

Another way of determining hydrogen peroxide concentrations is by performing a putative disproportionation reaction. This method uses a sulfite-reducing bacteria called ATP sulfurylase. This method of hydrogen peroxide decomposition is much more effective in determining molecular weight and structure. In this way, the reaction can be monitored continuously, allowing the researcher to compare the hydrogen peroxide rate with ammonia.
Disproportionation reaction in hydrogen peroxide
The disproportionation reaction in hydrogen peroxide is the process by which H2O2 is reacted with univalent oxygen to form water and oxygen. The oxygen atoms in hydrogen peroxide are in an oxidation state of -1, so they can either be oxidized to form O2 or reduced to water. The overall cell potential for the disproportionation reaction is positive, so this is the appropriate mechanism for the decomposition of H2O2.
The disproportionation reaction can be further broken down into the oxidation and reduction half-reactions. This is done to determine how many electrons are involved in each half-reaction. The oxidation half must be multiplied by 2 to achieve balance. The reduction half will involve 3 molecules and four electrons. Once you know the number of electrons involved in each half-reaction, you can calculate the balance of oxidation and reduction.
Effects of catalyst on the decomposition of hydrogen peroxide
The redox properties of hydrogen peroxide are well-known, as they can remove electrons from other substances and reduce inorganic ions. In its natural form, hydrogen peroxide decomposes into water and oxygen, releasing free radicals. The decomposition reaction occurs in the presence of a catalyst, a transition metal. Transition metals such as iron and manganese are found in nearly every living creature.
The mineralogical composition of the terrigenous rock plays a crucial role in decomposing hydrogen peroxide. Catalytic features of metal surfaces also play an essential role. Lastly, the salinity of the water plays a vital role in the process, as it enhances the pressure of the hydrogen peroxide. Hydrogen peroxide decomposition results in a significant increase in pressure within a reservoir.
A catalyst is needed to increase the rate of hydrogen peroxide decomposition. It is not part of the products of the chemical reaction but instead helps it happen faster. The catalyst does not change during the reaction, but it speeds it up by increasing the reaction rate. As a result, oxygen gas is released from hydrogen peroxide, and the catalyst helps the reaction take place faster.
The effects of catalysts on hydrogen peroxide degradation depend highly on the type of substrate used and the reaction conditions. Aluminum has a high starting activity, while tin decreases inhibition. The concentration of these metals significantly affects the rate of decomposition of hydrogen peroxide. The concentration of the catalyst determines the degree of inhibition and the start of the activity.
While using an elephant toothpaste demonstration, the catalyst helps to speed up the chemical reaction. The elephant toothpaste, for example, produces water vapor. The decomposition process is slow, but the presence of a catalyst speeds it up significantly. Likewise, adding a small amount of soap to the mixture speeds up the process, producing a large amount of foam. However, it is essential to include yeast in the product side of the chemical equation.
Several different types of catalysts have been identified as potential candidates for hydrogen peroxide decomposition. Potassium iodide is an example of a material that is a suitable catalyst. Potassium iodide is a catalyst because it produces the hypoiodite ion when it reacts with hydrogen peroxide and creates water.
The decomposition of H2O2 can be controlled by adjusting several variables. Temperature, concentration, pH, and the type of catalyst all significantly impact the final product. These variables are essential when considering the decomposition of H2O2 in the oil phase. However, the rate at which hydrogen peroxide enters the oil phase is limited. The higher the concentration of H2O2, the slower the decomposition process takes place.