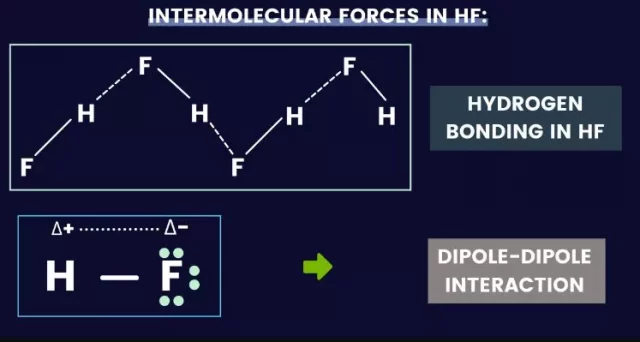
Types of HF Intermolecular Forces
You must now understand the three main categories of intermolecular forces. Which are: Van der Waals’ forces (London dispersal forces) Dipole-dipole forces that are constant Water Bonding Quick response: Hydrogen bonding is the main ” IMF ” in hydrogen fluoride (HF) (as hydrogen is bonded to fluorine).
The types of HF intermolecular forces are dipole-dipole attraction and London dispersion force. These forces affect the physical properties of a material and are determined by its vibrational frequency. The HF intermolecular forces are fragile but are essential for materials to maintain their properties. This article discusses the different types of HF intermolecular forces and their effects. In addition, it introduces the concept of hydrogen bonds. For example, fluorine has an electronegativity of 4.00 on the Pauling scale, while hydrogen has an electronegativity of 2.20 on the Pauling scale. As F is so much more electronegative than H, it attracts the bonding pair of electrons in the H-F covalent bond. This gives F a partial negative charge and H a partial positive charge. This polarizes the molecule. The positive H atom will then form weak bonds with the lone pairs of electrons on the F atom of another molecule. This is known as a hydrogen bond and is the most vital type of intermolecular bond. This gives HF an unusually high boiling point compared to other simple molecular compounds.
Dipole-dipole interactions
Hydrofluoric acid is a highly polar compound. Its H-F bond results in low electron density around the hydrogen atom, which aligns the molecules in a way that maximizes the attraction between polar atoms with opposite charges and minimizes the repulsion between polar atoms with similar charges. Because hydrogen has no inner electrons, its partial positive charge is quite strong and has a powerful interaction with lone pairs on fluorine atoms.
The energy of dipole-dipole interactions is proportional to 1/r3. When more than two molecules interact, the interaction’s energy is lessened by doubling the distance between the molecules. For example, a solid made of HCl and NaCl has a high melting point, but these are held together by dipole-dipole interactions.
Solid intermolecular forces can sometimes produce anomalously high melting and boiling points. The most vital intermolecular force is the hydrogen bond, while the weakest is the London dispersion force. As polar hydrogen is polar, it can approach another molecule much more closely than most other dipoles. The force of hydrogen bonds is responsible for many of the unusual properties of water.
Hydrogen fluoride exhibits strong hydrogen bonding between HF molecules. As a result, hydrogen fluoride has a higher boiling point than its molecular mass alone. While other hydrogen halides are gases at room temperatures, hydrogen fluoride is a liquid. This is because hydrogen fluoride molecules have strong electrostatic interactions with the other molecules. In addition, hydrogen fluoride molecules have higher boiling and melting points and more significant vaporization and fusion enthalpies than nonpolar compounds.

Hydrofluoride, for example, has a solid dipole-dipole interaction. The two substances are attracted to each other. It is the most vital intermolecular force present in each substance. However, dipole-dipole interactions between hydrogen fluoride and water are weaker. The stronger the interactions between the two substances, the stronger they are. So, it is essential to note that weaker dipole-dipole interactions in hf compounds are weaker than those among polar and nonpolar substances.
London dispersion forces
The difference between the London dispersion forces and HF (hydrogen bonding) intermolecular force is significant because these two interactions differ considerably. London dispersion forces occur between any two molecules, but they are weaker than the dipole-dipole forces. This is because the London dispersion forces are caused by random electron movements rather than hydrogen bonds or dipole-dipole interactions.
The forces that hold two molecules together depend on the attraction of their opposite charges. This attraction is weak and falls off rapidly as the distance increases. London showed with quantum mechanics that the attractive energy of two molecules falls off at a rate of 1/r6. This means that for every double distance, the attractive energy of two molecules decreases by about 26 and 64, respectively. As a result, it is possible to break the London dispersion forces without too much energy.
Dipole-dipole interactions occur when a molecule’s positive and negative ends have a dipole, causing an electrostatic attraction between them. These interactions’ strength depends on the molecule’s number of electrons and surface area. In addition, dispersion forces between molecules are more potent than Keesom forces and are the second most potent van der Waals forces. The strength of London dispersion forces is proportional to the molar mass of the molecules, and the larger the atoms, the stronger the dispersion force.
Hydrogen fluoride exhibits London dispersion forces and dipole-dipole interaction. In contrast to its electronegative counterpart, hydrogen fluoride is a polar molecule. It has Dipole-Dipole, Van der Waals’, and London dispersion forces. Each molecule has its own electronegativity; the greater the value, the higher the electronegativity.
Hydrofluoride exhibits strong hydrogen bonding between HF molecules. Its boiling point is higher than the boiling point of either of its constituent parts alone. Hydrofluoride and ammonia are gases at room temperatures. The dissociation equation between the two is H+ + F-. The strength of the hydrogen-fluoride bonding is also higher than that of the hydrogen-fluoride molecule.
Hydrogen bonding
HF forms hydrogen bonds, which have a higher boiling point than water. It is a member of the same group as fluorine in the periodic table but is heavier and less electronegative than fluorine. Unlike most halides, HF is a liquid at room temperature. The difference in electronegativity between hydrogen and fluorine is the largest of any group. One reason is the atomic size difference. Another reason is that fluorine does not form hydrogen bonds.
The depth of interaction potential De determines whether a hydrogen bond is strong or weak. Most textbooks define hydrogen bonds as between hydrogen atoms in ionic compounds. Hydrogen must bond to a substantially more electronegative atom in a covalent system. The distance between the two atoms is typically between 2.8 and 3.1 A. These parameters are consistent with the hydrogen bond’s electrostatic nature.
The lone pair effect also occurs in water. This attraction is similar to the covalent bond between two molecules but more potent than the ordinary dipole-dipole interaction. The strength of hydrogen bonds is one-tenth of the average covalent bond. In liquid water, hydrogen bonds are broken constantly. Thus, the covalent bond between hydrogen and oxygen is equivalent to an unbreakable marriage, while the hydrogen-oxygen bond is similar to “just good friends.”
The most vital intermolecular force is hydrogen bonding. Chan (2003) conducted a study to investigate the correlation between students’ hydrogen bonding and physical properties. He conducted an interview, administered a free response test, and gave them examples of water and iodine molecules at room temperature. Chan found a strong correlation between hydrogen bonding and water temperature.
Despite the differences between hydrogen-bonding and covalent bonds, the interaction between the hydrogen and fluorine molecules is the strongest between any two molecules. This force depends on the distance between the two molecules, the angle at which the molecules are in contact, and the extent of the network. Hydrogen bonds are strong because hydrogen molecules have two hydrogen atoms, while fluorine bonds only one.
Effects on material properties
The strength of the intermolecular forces (IMFs) between two molecules of a substance will determine its physical properties. Weak forces cause particles to move apart, while strong forces make the molecules stick together in a solid structure. Moreover, intermolecular forces are affected by temperature. Therefore, intermolecular forces affect the physical properties of materials, such as melting and boiling points.
Strong intermolecular forces between two molecules produce anomalously high melting and boiling points. These properties are due to hydrogen being a highly polar atom with a large partial positive charge. Other atoms have hefty negative charges. In addition, the H-O, F, and N-F bonds are substantial because of the nature of hydrogen. This allows them to approach each other closer than other dipoles.
Another critical aspect of intermolecular forces is their effect on surface energy. They can affect a material’s surface tension, temperature, and elasticity. For example, a solid can expand when pressure is increased. A liquid can shrink when pressure increases, and a solid can expand or contract when pressure is reduced. However, fluid can deform because of the effects of intermolecular forces.
Strong intermolecular forces, in contrast, are also a significant factor in the formation of crystals. The intermolecular forces that hold two molecules together are called Van der Waals forces. The strength of intermolecular forces is directly proportional to the amount of kinetic energy contained in the material. This is also reflected in the polarity of the atoms. For example, solids have strong intermolecular forces, while liquids and gases have negligible interactions between molecules.
All molecules exhibit a dispersion force. The stronger the intermolecular forces are, the more rigid the substance will be. Solids are solids because they have the highest intermolecular forces. They are rigid and have a fixed volume and shape. Their dispersion forces scale with molecular size, but they are the most critical factor in determining a material’s properties.
Types of HF Intermolecular Forces
You must now understand the three main categories of intermolecular forces. Which are: Van der Waals’ forces (London dispersal forces) Dipole-dipole forces that are constant Water Bonding Quick response: Hydrogen bonding is the main ” IMF ” in hydrogen fluoride (HF) (as hydrogen is bonded to fluorine).
The types of HF intermolecular forces are dipole-dipole attraction and London dispersion force. These forces affect the physical properties of a material and are determined by its vibrational frequency. The HF intermolecular forces are fragile but are essential for materials to maintain their properties. This article discusses the different types of HF intermolecular forces and their effects. In addition, it introduces the concept of hydrogen bonds. For example, fluorine has an electronegativity of 4.00 on the Pauling scale, while hydrogen has an electronegativity of 2.20 on the Pauling scale. As F is so much more electronegative than H, it attracts the bonding pair of electrons in the H-F covalent bond. This gives F a partial negative charge and H a partial positive charge. This polarizes the molecule. The positive H atom will then form weak bonds with the lone pairs of electrons on the F atom of another molecule. This is known as a hydrogen bond and is the most vital type of intermolecular bond. This gives HF an unusually high boiling point compared to other simple molecular compounds.
Dipole-dipole interactions
Hydrofluoric acid is a highly polar compound. Its H-F bond results in low electron density around the hydrogen atom, which aligns the molecules in a way that maximizes the attraction between polar atoms with opposite charges and minimizes the repulsion between polar atoms with similar charges. Because hydrogen has no inner electrons, its partial positive charge is quite strong and has a powerful interaction with lone pairs on fluorine atoms.
The energy of dipole-dipole interactions is proportional to 1/r3. When more than two molecules interact, the interaction’s energy is lessened by doubling the distance between the molecules. For example, a solid made of HCl and NaCl has a high melting point, but these are held together by dipole-dipole interactions.
Solid intermolecular forces can sometimes produce anomalously high melting and boiling points. The most vital intermolecular force is the hydrogen bond, while the weakest is the London dispersion force. As polar hydrogen is polar, it can approach another molecule much more closely than most other dipoles. The force of hydrogen bonds is responsible for many of the unusual properties of water.
Hydrogen fluoride exhibits strong hydrogen bonding between HF molecules. As a result, hydrogen fluoride has a higher boiling point than its molecular mass alone. While other hydrogen halides are gases at room temperatures, hydrogen fluoride is a liquid. This is because hydrogen fluoride molecules have strong electrostatic interactions with the other molecules. In addition, hydrogen fluoride molecules have higher boiling and melting points and more significant vaporization and fusion enthalpies than nonpolar compounds.

Hydrofluoride, for example, has a solid dipole-dipole interaction. The two substances are attracted to each other. It is the most vital intermolecular force present in each substance. However, dipole-dipole interactions between hydrogen fluoride and water are weaker. The stronger the interactions between the two substances, the stronger they are. So, it is essential to note that weaker dipole-dipole interactions in hf compounds are weaker than those among polar and nonpolar substances.
London dispersion forces
The difference between the London dispersion forces and HF (hydrogen bonding) intermolecular force is significant because these two interactions differ considerably. London dispersion forces occur between any two molecules, but they are weaker than the dipole-dipole forces. This is because the London dispersion forces are caused by random electron movements rather than hydrogen bonds or dipole-dipole interactions.
The forces that hold two molecules together depend on the attraction of their opposite charges. This attraction is weak and falls off rapidly as the distance increases. London showed with quantum mechanics that the attractive energy of two molecules falls off at a rate of 1/r6. This means that for every double distance, the attractive energy of two molecules decreases by about 26 and 64, respectively. As a result, it is possible to break the London dispersion forces without too much energy.
Dipole-dipole interactions occur when a molecule’s positive and negative ends have a dipole, causing an electrostatic attraction between them. These interactions’ strength depends on the molecule’s number of electrons and surface area. In addition, dispersion forces between molecules are more potent than Keesom forces and are the second most potent van der Waals forces. The strength of London dispersion forces is proportional to the molar mass of the molecules, and the larger the atoms, the stronger the dispersion force.
Hydrogen fluoride exhibits London dispersion forces and dipole-dipole interaction. In contrast to its electronegative counterpart, hydrogen fluoride is a polar molecule. It has Dipole-Dipole, Van der Waals’, and London dispersion forces. Each molecule has its own electronegativity; the greater the value, the higher the electronegativity.
Hydrofluoride exhibits strong hydrogen bonding between HF molecules. Its boiling point is higher than the boiling point of either of its constituent parts alone. Hydrofluoride and ammonia are gases at room temperatures. The dissociation equation between the two is H+ + F-. The strength of the hydrogen-fluoride bonding is also higher than that of the hydrogen-fluoride molecule.
Hydrogen bonding
HF forms hydrogen bonds, which have a higher boiling point than water. It is a member of the same group as fluorine in the periodic table but is heavier and less electronegative than fluorine. Unlike most halides, HF is a liquid at room temperature. The difference in electronegativity between hydrogen and fluorine is the largest of any group. One reason is the atomic size difference. Another reason is that fluorine does not form hydrogen bonds.
The depth of interaction potential De determines whether a hydrogen bond is strong or weak. Most textbooks define hydrogen bonds as between hydrogen atoms in ionic compounds. Hydrogen must bond to a substantially more electronegative atom in a covalent system. The distance between the two atoms is typically between 2.8 and 3.1 A. These parameters are consistent with the hydrogen bond’s electrostatic nature.
The lone pair effect also occurs in water. This attraction is similar to the covalent bond between two molecules but more potent than the ordinary dipole-dipole interaction. The strength of hydrogen bonds is one-tenth of the average covalent bond. In liquid water, hydrogen bonds are broken constantly. Thus, the covalent bond between hydrogen and oxygen is equivalent to an unbreakable marriage, while the hydrogen-oxygen bond is similar to “just good friends.”
The most vital intermolecular force is hydrogen bonding. Chan (2003) conducted a study to investigate the correlation between students’ hydrogen bonding and physical properties. He conducted an interview, administered a free response test, and gave them examples of water and iodine molecules at room temperature. Chan found a strong correlation between hydrogen bonding and water temperature.
Despite the differences between hydrogen-bonding and covalent bonds, the interaction between the hydrogen and fluorine molecules is the strongest between any two molecules. This force depends on the distance between the two molecules, the angle at which the molecules are in contact, and the extent of the network. Hydrogen bonds are strong because hydrogen molecules have two hydrogen atoms, while fluorine bonds only one.
Effects on material properties
The strength of the intermolecular forces (IMFs) between two molecules of a substance will determine its physical properties. Weak forces cause particles to move apart, while strong forces make the molecules stick together in a solid structure. Moreover, intermolecular forces are affected by temperature. Therefore, intermolecular forces affect the physical properties of materials, such as melting and boiling points.
Strong intermolecular forces between two molecules produce anomalously high melting and boiling points. These properties are due to hydrogen being a highly polar atom with a large partial positive charge. Other atoms have hefty negative charges. In addition, the H-O, F, and N-F bonds are substantial because of the nature of hydrogen. This allows them to approach each other closer than other dipoles.
Another critical aspect of intermolecular forces is their effect on surface energy. They can affect a material’s surface tension, temperature, and elasticity. For example, a solid can expand when pressure is increased. A liquid can shrink when pressure increases, and a solid can expand or contract when pressure is reduced. However, fluid can deform because of the effects of intermolecular forces.
Strong intermolecular forces, in contrast, are also a significant factor in the formation of crystals. The intermolecular forces that hold two molecules together are called Van der Waals forces. The strength of intermolecular forces is directly proportional to the amount of kinetic energy contained in the material. This is also reflected in the polarity of the atoms. For example, solids have strong intermolecular forces, while liquids and gases have negligible interactions between molecules.
All molecules exhibit a dispersion force. The stronger the intermolecular forces are, the more rigid the substance will be. Solids are solids because they have the highest intermolecular forces. They are rigid and have a fixed volume and shape. Their dispersion forces scale with molecular size, but they are the most critical factor in determining a material’s properties.