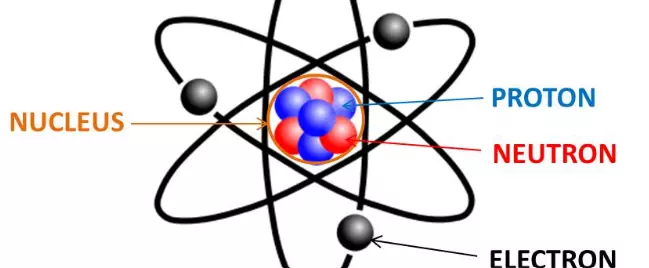
Where Are Neutrons and Protons Located in an Atom?
In an atom, there are three particles: Protons, Neutrons, and Electrons. Protons are located in the nucleus, the tiny dense region at the center of the atom. Protons are charged positively with a positive electric charge of one (+1). Their mass is one atomic mass unit (amu), or 1.67 kilograms. Protons comprise almost all of the mass of an atom.
Neutrons have a neutral charge.
What is an atom? Atoms are small units of matter that contain protons and electrons. These atoms are in equilibrium when they have an equal number of protons and electrons. This is referred to as neutrality. Neutrons have a net negative charge, while protons are positively charged. The negative charge of electrons contributes to the charge of an atom. Neutral atoms have the same number of protons and electrons, and the overall charge is neutral.
The proton has two electrons and one down quark. This made the proton positively charged throughout. However, the middle area had a higher charge than the interior and exterior. In an atom with only neutrons, the charge on a neutron is negative, so it is neutral. Because the electron and proton are the same sizes, their charge cancels each other.
Unlike protons, neutrons have no net electrical charge. However, they are bonded with the protons with strong residual force. As such, they are referred to as ions. An atom can also be characterized by its number of electrons. In this case, the amount of electrons is two times greater than the number of protons. In this way, the net charge is positive, which means that there is one more electron than a molecule’s mass.
They have the same mass as protons.
Protons and neutrons have equal masses, but electrons are lighter. The mass of a neutron is only 0.054% of the mass of a proton. This difference between protons and electrons makes it easy to see how they differ. While protons and electrons are similar in appearance, they are significantly different in mass and charge. They would be much heavier than one another if they had the same mass.
A neutron’s and proton’s mass is the same, but their properties differ. The interior of a neutron is similar to that of a proton, although neutrons contain different types of quarks. This difference in composition is fundamental to atomic physics. However, it may not be evident to an outside observer. Therefore, scientists are constantly working on finding ways to make this difference clearer.
In atoms, neutrons and protons are found inside the nucleus, which is the center of an atom. Because the positive and negative charges on each particle attract each other, electrons move through space. In simpler versions, electrons are seen as particles orbiting the nucleus, but in reality, they are much more complex. While they are roughly the same as protons in an atom, their mass is nearly half that of a proton.
They exchange mesons with protons to form a strong nuclear force.
The strong nuclear force is a powerful attraction between two charged particles. Neutrons and protons can be found in all atoms, and each proton has a positive charge. Because the protons must be close to each other to interact, they must exchange mesons. These exchanges of mesons are like the constant hitting of tennis or ping pong balls. Because protons must be close to each other, a strong nuclear force is necessary to hold two nucleons together.
Neutrons and protons exchange mesons to form a strong nuclear force. The interaction between these particles is powerful and has an exquisitely fine-tuned nature—neutrons, the lightest strongly interacting particles, exchange mesons with protons. Heavy mesons, such as pions and pi-mesons form the short-range version of the strong nuclear force.
The strong nuclear force can only couple with colored particles, not externally white ones. For these particles to interact with each other, they exchange mesons. The lightest meson is the pion, which is about 1.4 times lighter than a proton. The electromagnetic force and gravitational force have infinite ranges. A weaker, more subtle interaction between two particles can be a couple of meters apart.
They interact with electrons to create chemical bonds.
Neutrons interact with electrons to form chemical bonds. When interacting with an atom, they tend to spend more time with it. For example, electrons spend more time in the water with oxygen because oxygen has a greater electron affinity than hydrogen. This makes the H-bond slightly negative while leaving the hydrogen atom slightly positive. Hence, water is polar, and an ionic bond would be formed between oxygen and hydrogen atoms.
The configuration of the electrons in an atom is responsible for most of the chemical properties of an atom. An atom’s chemical properties are determined by its electron configuration and the number of protons in the heavy nucleus, but the number of neutrons, on the other hand, does not have any effect on the electron configuration. The mass of an atom is the sum of its atomic and neutron number.
The neutron, the electron, and the proton’s spin must be fractional to form a chemical bond. Because of their small size and spin, they are supposed to bond with one another to form a neutron. However, this is not the case. The electron and proton spins cannot merge into one neutron. This is why the nuclear magneton is needed to form chemical bonds.
They undergo fission when bombarded by heavy elements.
Heavy elements with smaller binding energies can decay into lighter, intermediate-mass nuclei. These elements are at or near the peak of the binding energy graph near 56. As these heavy elements decay, neutrons are also produced. This process is known as fission. This reaction breaks up a large nucleus into smaller pieces. This process is most often induced by bombarding heavy elements with neutrons.
Nuclear fission was first reported in 1939 by German physicists. They bombarded uranium-235 atoms with slow-moving neutrons, splitting them into smaller fragments. These fragments each contained several neutrons and were middle-of-the-period table elements. Since then, fission has been reported for many other isotopes, including helium. Most actinides have an odd number of neutrons. The typical nuclear fission reaction is illustrated in Figure 2.
When bombarded by heavy elements, neutrons undergo fission. Heavy elements that are not inert are unstable, and a nuclear reaction occurs when the neutrons strike one another. The nuclear reaction is the primary source of energy in atomic weapons. This process allows for massive destruction. The destruction of a nuclear device is called a “nuclear explosion.”
They orbit the nucleus.
When the electrons orbit the nucleus in an atomic structure, they move around the atom at light speed. They are found in different levels, called orbitals, around the nucleus. An electron can be in any orbital, which is why they are sometimes referred to as spheres, lobes, or doughnuts. To describe how electrons move, it’s helpful to use an analogy to understand how energy is stored in an atom.
A single atom consists of three constituent particles: protons, neutrons, and electrons. Protons have a positive charge, while neutrons have no charge. The electrons orbit the nucleus due to the force of attraction between them and the nucleus. The electrons move around the nucleus because they are less massive than the protons and neutrons. The nucleus has the most mass, but the electrons have a negative charge.
The distance of an electron from the nucleus varies depending on the energy level. The nucleus can be located at the lower energy level along the atom’s surface. However, if the electrons orbit in the middle of the sphere, they will have a higher energy level. The electrons will have more energy than the protons but will not be as dense as the nucleus.
They are composites of extremely small elementary particles.
The two microscopic elementary particles that make up an atom are protons and neutrons. They are composed of one up and two down quarks and are held together by the strong nuclear force, one of the four fundamental forces of nature. This force counteracts the positive-charged protons’ tendency to repel each other. This force allows the nucleus to remain together, even though quarks of other types are also present.
Scientists had theorized that there was more than one fundamental particle in an atom, but they assumed that the size of the unit was the size of the smallest atomic particle, hydrogen. In 1897, J.J. Thompson announced the discovery of a unit that was 1000 times smaller than a hydrogen atom and 1800 times lighter. Scientists have since studied the properties of neutrons and their role in the structure of an atom.
Because of their size, bosons and neutrons are nature’s most common types of elementary particles. Their range of sizes is so extensive that a radio wave photon can stretch for miles. On the other hand, fermions are tiny and so small that current experiments have only put an upper limit on the sizes of these particles. A proton is about one-billionth of an atom’s diameter, while a neutron is about ten thousand times smaller.
Where Are Neutrons and Protons Located in an Atom?
In an atom, there are three particles: Protons, Neutrons, and Electrons. Protons are located in the nucleus, the tiny dense region at the center of the atom. Protons are charged positively with a positive electric charge of one (+1). Their mass is one atomic mass unit (amu), or 1.67 kilograms. Protons comprise almost all of the mass of an atom.
Neutrons have a neutral charge.
What is an atom? Atoms are small units of matter that contain protons and electrons. These atoms are in equilibrium when they have an equal number of protons and electrons. This is referred to as neutrality. Neutrons have a net negative charge, while protons are positively charged. The negative charge of electrons contributes to the charge of an atom. Neutral atoms have the same number of protons and electrons, and the overall charge is neutral.
The proton has two electrons and one down quark. This made the proton positively charged throughout. However, the middle area had a higher charge than the interior and exterior. In an atom with only neutrons, the charge on a neutron is negative, so it is neutral. Because the electron and proton are the same sizes, their charge cancels each other.
Unlike protons, neutrons have no net electrical charge. However, they are bonded with the protons with strong residual force. As such, they are referred to as ions. An atom can also be characterized by its number of electrons. In this case, the amount of electrons is two times greater than the number of protons. In this way, the net charge is positive, which means that there is one more electron than a molecule’s mass.
They have the same mass as protons.
Protons and neutrons have equal masses, but electrons are lighter. The mass of a neutron is only 0.054% of the mass of a proton. This difference between protons and electrons makes it easy to see how they differ. While protons and electrons are similar in appearance, they are significantly different in mass and charge. They would be much heavier than one another if they had the same mass.
A neutron’s and proton’s mass is the same, but their properties differ. The interior of a neutron is similar to that of a proton, although neutrons contain different types of quarks. This difference in composition is fundamental to atomic physics. However, it may not be evident to an outside observer. Therefore, scientists are constantly working on finding ways to make this difference clearer.
In atoms, neutrons and protons are found inside the nucleus, which is the center of an atom. Because the positive and negative charges on each particle attract each other, electrons move through space. In simpler versions, electrons are seen as particles orbiting the nucleus, but in reality, they are much more complex. While they are roughly the same as protons in an atom, their mass is nearly half that of a proton.
They exchange mesons with protons to form a strong nuclear force.
The strong nuclear force is a powerful attraction between two charged particles. Neutrons and protons can be found in all atoms, and each proton has a positive charge. Because the protons must be close to each other to interact, they must exchange mesons. These exchanges of mesons are like the constant hitting of tennis or ping pong balls. Because protons must be close to each other, a strong nuclear force is necessary to hold two nucleons together.
Neutrons and protons exchange mesons to form a strong nuclear force. The interaction between these particles is powerful and has an exquisitely fine-tuned nature—neutrons, the lightest strongly interacting particles, exchange mesons with protons. Heavy mesons, such as pions and pi-mesons form the short-range version of the strong nuclear force.
The strong nuclear force can only couple with colored particles, not externally white ones. For these particles to interact with each other, they exchange mesons. The lightest meson is the pion, which is about 1.4 times lighter than a proton. The electromagnetic force and gravitational force have infinite ranges. A weaker, more subtle interaction between two particles can be a couple of meters apart.
They interact with electrons to create chemical bonds.
Neutrons interact with electrons to form chemical bonds. When interacting with an atom, they tend to spend more time with it. For example, electrons spend more time in the water with oxygen because oxygen has a greater electron affinity than hydrogen. This makes the H-bond slightly negative while leaving the hydrogen atom slightly positive. Hence, water is polar, and an ionic bond would be formed between oxygen and hydrogen atoms.
The configuration of the electrons in an atom is responsible for most of the chemical properties of an atom. An atom’s chemical properties are determined by its electron configuration and the number of protons in the heavy nucleus, but the number of neutrons, on the other hand, does not have any effect on the electron configuration. The mass of an atom is the sum of its atomic and neutron number.
The neutron, the electron, and the proton’s spin must be fractional to form a chemical bond. Because of their small size and spin, they are supposed to bond with one another to form a neutron. However, this is not the case. The electron and proton spins cannot merge into one neutron. This is why the nuclear magneton is needed to form chemical bonds.
They undergo fission when bombarded by heavy elements.
Heavy elements with smaller binding energies can decay into lighter, intermediate-mass nuclei. These elements are at or near the peak of the binding energy graph near 56. As these heavy elements decay, neutrons are also produced. This process is known as fission. This reaction breaks up a large nucleus into smaller pieces. This process is most often induced by bombarding heavy elements with neutrons.
Nuclear fission was first reported in 1939 by German physicists. They bombarded uranium-235 atoms with slow-moving neutrons, splitting them into smaller fragments. These fragments each contained several neutrons and were middle-of-the-period table elements. Since then, fission has been reported for many other isotopes, including helium. Most actinides have an odd number of neutrons. The typical nuclear fission reaction is illustrated in Figure 2.
When bombarded by heavy elements, neutrons undergo fission. Heavy elements that are not inert are unstable, and a nuclear reaction occurs when the neutrons strike one another. The nuclear reaction is the primary source of energy in atomic weapons. This process allows for massive destruction. The destruction of a nuclear device is called a “nuclear explosion.”
They orbit the nucleus.
When the electrons orbit the nucleus in an atomic structure, they move around the atom at light speed. They are found in different levels, called orbitals, around the nucleus. An electron can be in any orbital, which is why they are sometimes referred to as spheres, lobes, or doughnuts. To describe how electrons move, it’s helpful to use an analogy to understand how energy is stored in an atom.
A single atom consists of three constituent particles: protons, neutrons, and electrons. Protons have a positive charge, while neutrons have no charge. The electrons orbit the nucleus due to the force of attraction between them and the nucleus. The electrons move around the nucleus because they are less massive than the protons and neutrons. The nucleus has the most mass, but the electrons have a negative charge.
The distance of an electron from the nucleus varies depending on the energy level. The nucleus can be located at the lower energy level along the atom’s surface. However, if the electrons orbit in the middle of the sphere, they will have a higher energy level. The electrons will have more energy than the protons but will not be as dense as the nucleus.
They are composites of extremely small elementary particles.
The two microscopic elementary particles that make up an atom are protons and neutrons. They are composed of one up and two down quarks and are held together by the strong nuclear force, one of the four fundamental forces of nature. This force counteracts the positive-charged protons’ tendency to repel each other. This force allows the nucleus to remain together, even though quarks of other types are also present.
Scientists had theorized that there was more than one fundamental particle in an atom, but they assumed that the size of the unit was the size of the smallest atomic particle, hydrogen. In 1897, J.J. Thompson announced the discovery of a unit that was 1000 times smaller than a hydrogen atom and 1800 times lighter. Scientists have since studied the properties of neutrons and their role in the structure of an atom.
Because of their size, bosons and neutrons are nature’s most common types of elementary particles. Their range of sizes is so extensive that a radio wave photon can stretch for miles. On the other hand, fermions are tiny and so small that current experiments have only put an upper limit on the sizes of these particles. A proton is about one-billionth of an atom’s diameter, while a neutron is about ten thousand times smaller.